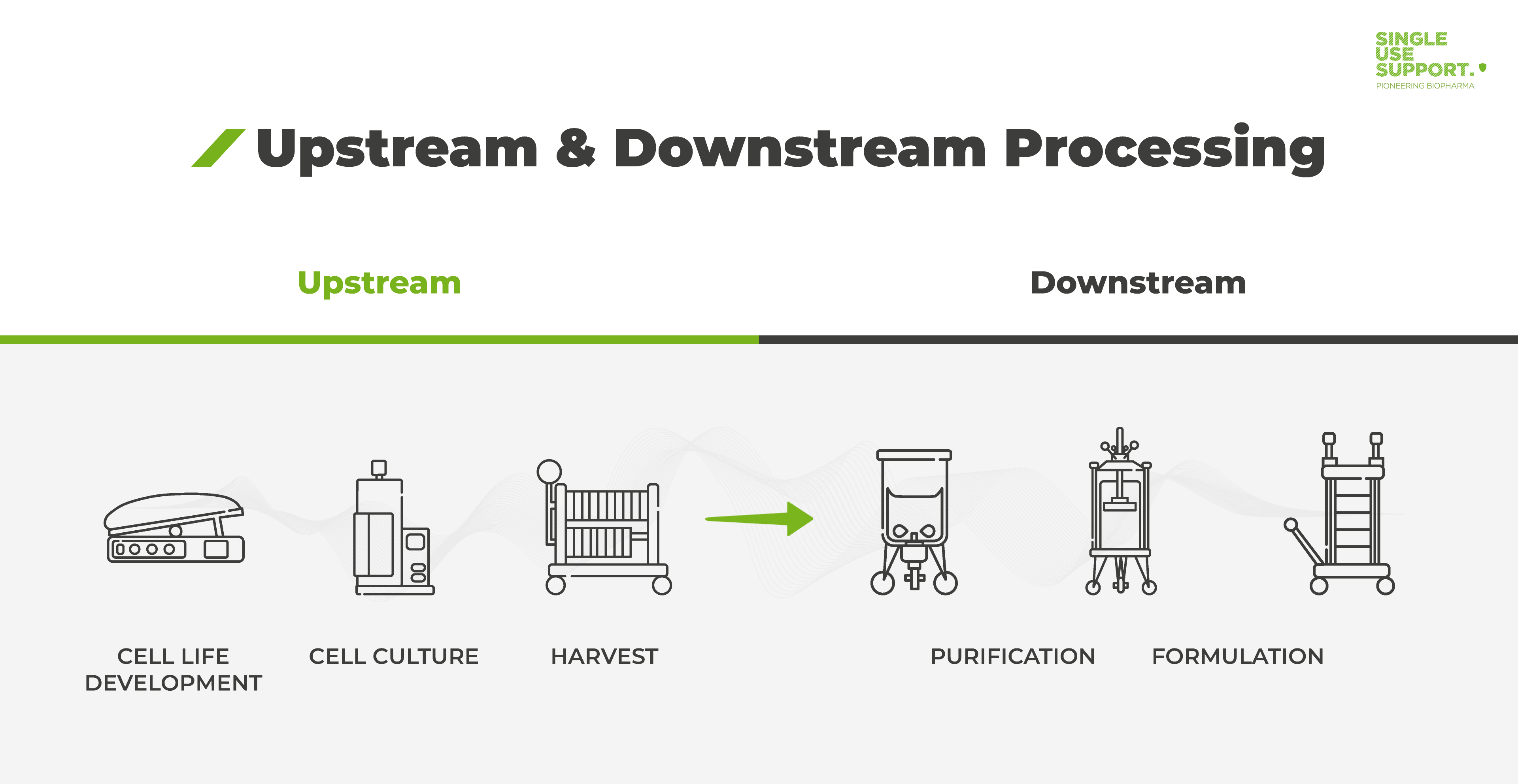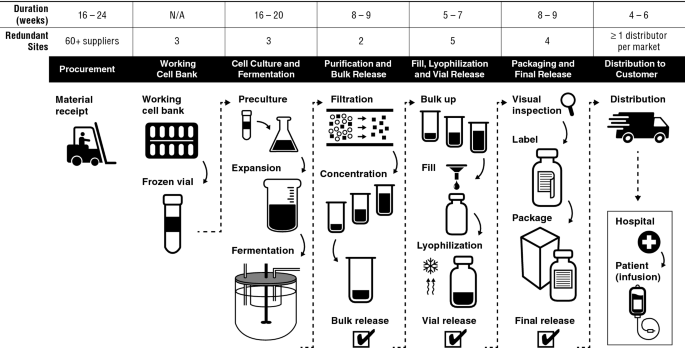
Ensuring Product Quality, Consistency and Patient Supply over Time for a Large-Volume Biologic: Experience with Remicade® | SpringerLink

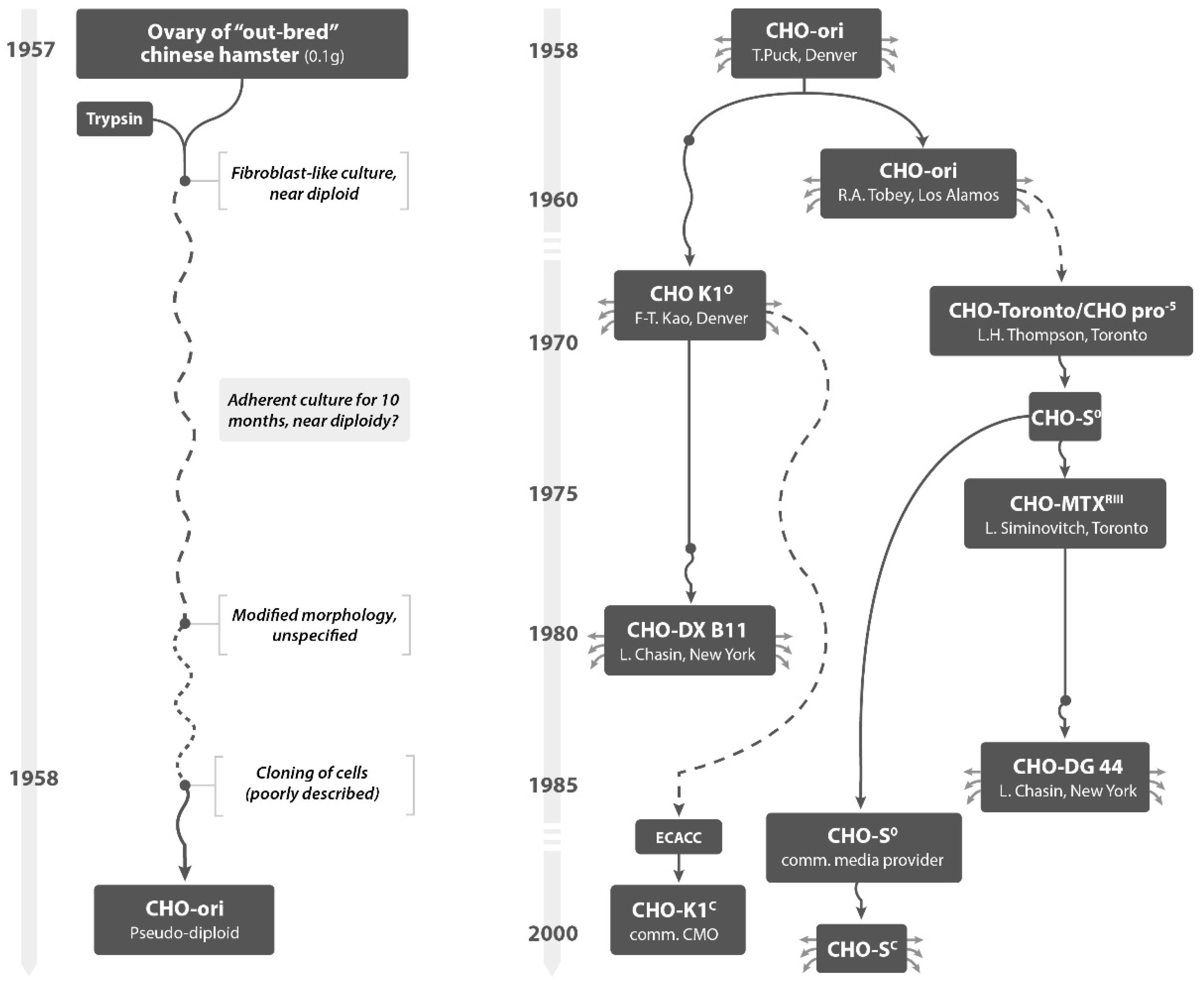
Figure 16 from Mise en place et développement d'une plateforme de culture pour la levure pichia pastoris | Semantic Scholar

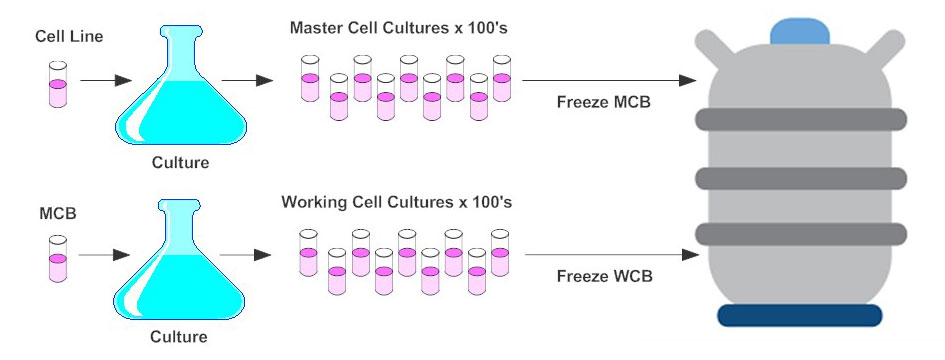
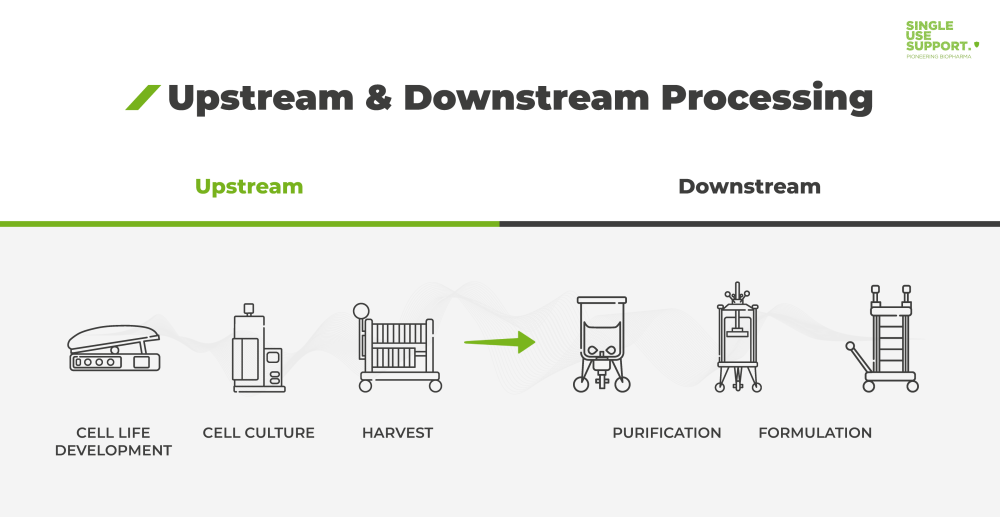
Biopharmaceutical Fermentation L8 9 S - Biopharmaceutical Fermentation. Lectures 8 and 9: Cell - Studocu

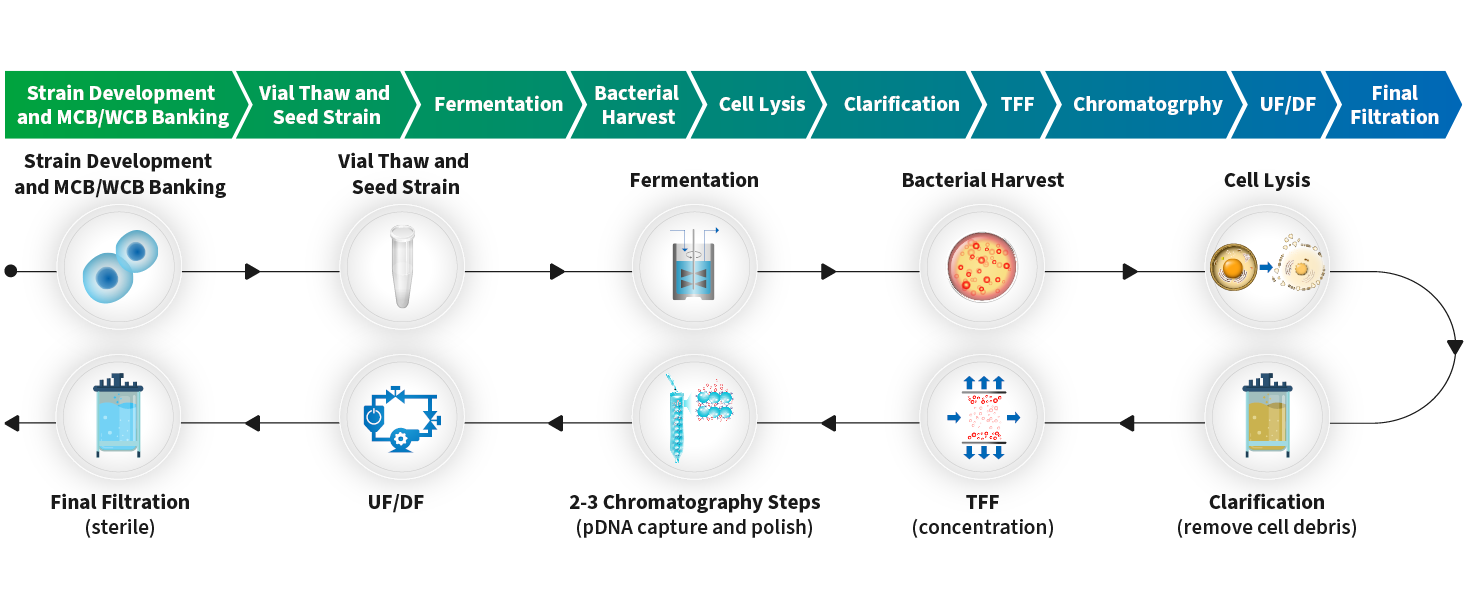
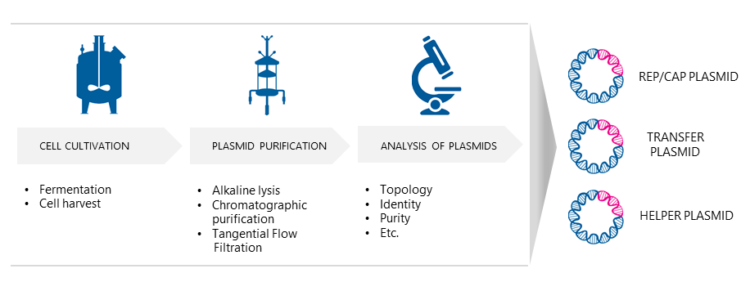
Phase-Dependent Approaches to Plasmid DNA Manufacture: Cobra Biologics describes how new guidelines for starting materials benefit developers of vectors for gene-based advanced therapy medicinal products: Genetic Engineering & Biotechnology News: Vol 41,