
An Unsaturated Nickel(0) NHC Catalyst: Facile Preparation and Structure of Ni(0)(NHC)2, Featuring a Reduction Process from Ni(II)(NHC)(acac)2 | Organometallics

Redox‐Neutral Nickel(II) Catalysis: Hydroarylation of Unactivated Alkenes with Arylboronic Acids - Wang - 2020 - Angewandte Chemie - Wiley Online Library

Redox‐Neutral Nickel(II) Catalysis: Hydroarylation of Unactivated Alkenes with Arylboronic Acids - Wang - 2020 - Angewandte Chemie - Wiley Online Library

Synthesis and structural characterization of nickel(II) complexes of 20-membered macrocyclic rings bearing chelating bis(N-heterocyclic carbene) ligands - ScienceDirect

An Unsaturated Nickel(0) NHC Catalyst: Facile Preparation and Structure of Ni(0)(NHC)2, Featuring a Reduction Process from Ni(II)(NHC)(acac)2 | Organometallics
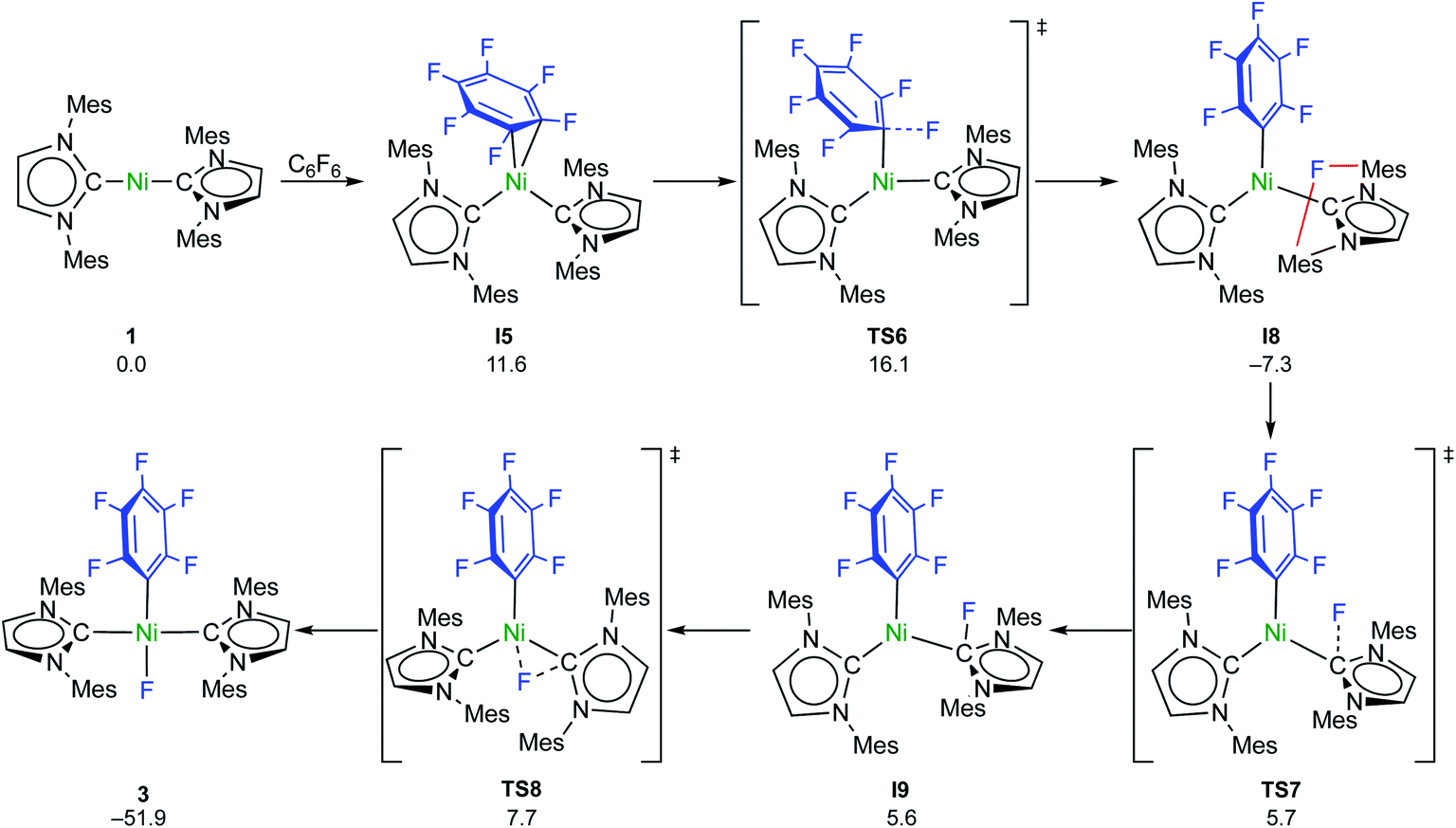
Coligand role in the NHC nickel catalyzed C–F bond activation: investigations on the insertion of bis(NHC) nickel into the C–F bond of hexafluorobenze ... - Chemical Science (RSC Publishing) DOI:10.1039/D0SC04237D

Well-defined nickel(II) tetrazole-saccharinate complex as homogeneous catalyst on the reduction of aldehydes: scope and reaction mechanism
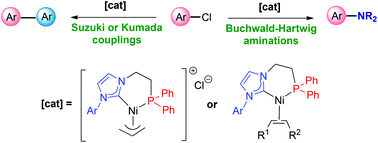
Phosphine-functionalized NHC Ni(ii) and Ni(0) complexes: synthesis, characterization and catalytic properties - Dalton Transactions (RSC Publishing)
Syntheses of Four-Coordinate Nickel(II)-Phosphine Compounds and a Rapid Suzuki–Miyaura Cross-Coupling Reaction for Short Labor

A surprising mechanism lacking the Ni(0) state during the Ni(II)-catalyzed P–C cross-coupling reaction performed in the absence of a reducing agent – An experimental and a theoretical study

An Unsaturated Nickel(0) NHC Catalyst: Facile Preparation and Structure of Ni(0)(NHC)2, Featuring a Reduction Process from Ni(II)(NHC)(acac)2 | Organometallics

Redox‐Neutral Nickel(II) Catalysis: Hydroarylation of Unactivated Alkenes with Arylboronic Acids - Wang - 2020 - Angewandte Chemie - Wiley Online Library

A surprising mechanism lacking the Ni(0) state during the Ni(II)-catalyzed P–C cross-coupling reaction performed in the absence of a reducing agent – An experimental and a theoretical study

The Synthesis and Catalytic Activity of New Mixed NHC-Phosphite Nickel(0) Complexes | Organometallics
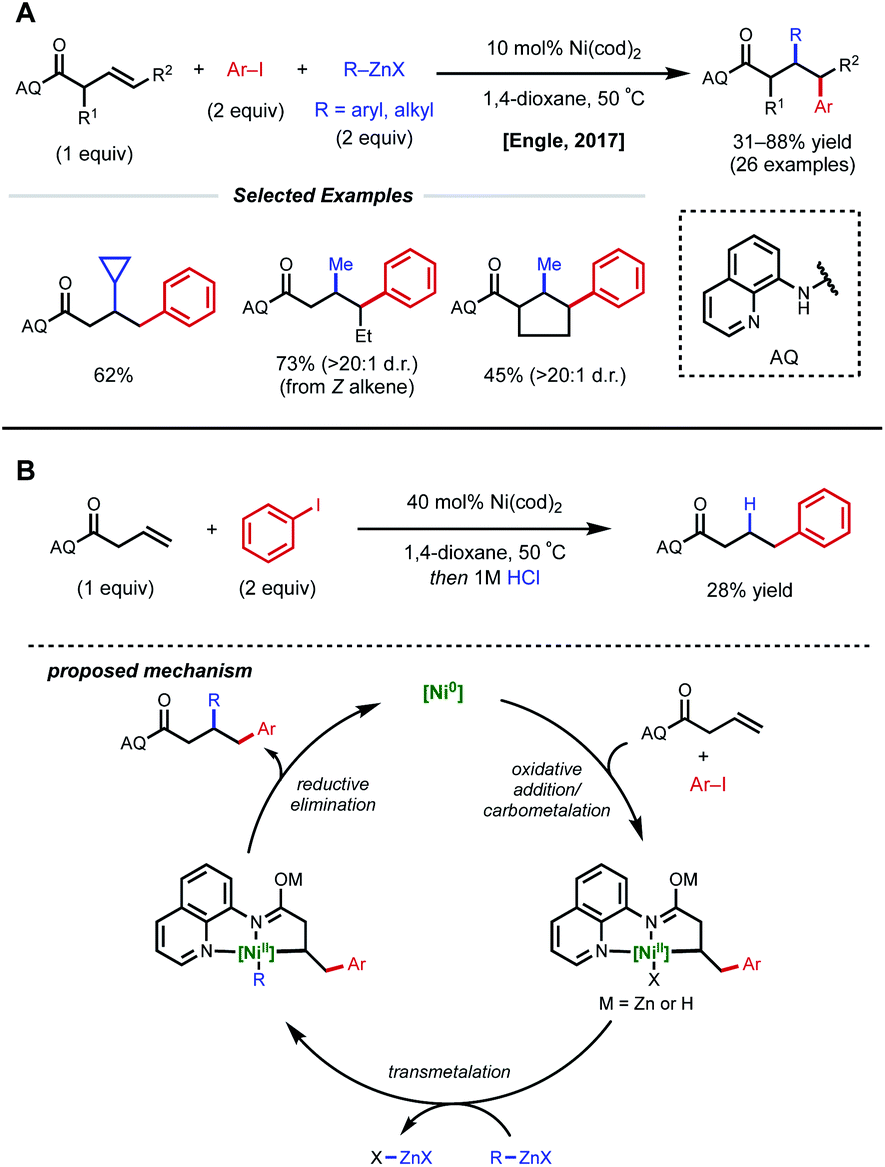
Recent developments in nickel-catalyzed intermolecular dicarbofunctionalization of alkenes - Chemical Science (RSC Publishing) DOI:10.1039/C9SC06006E
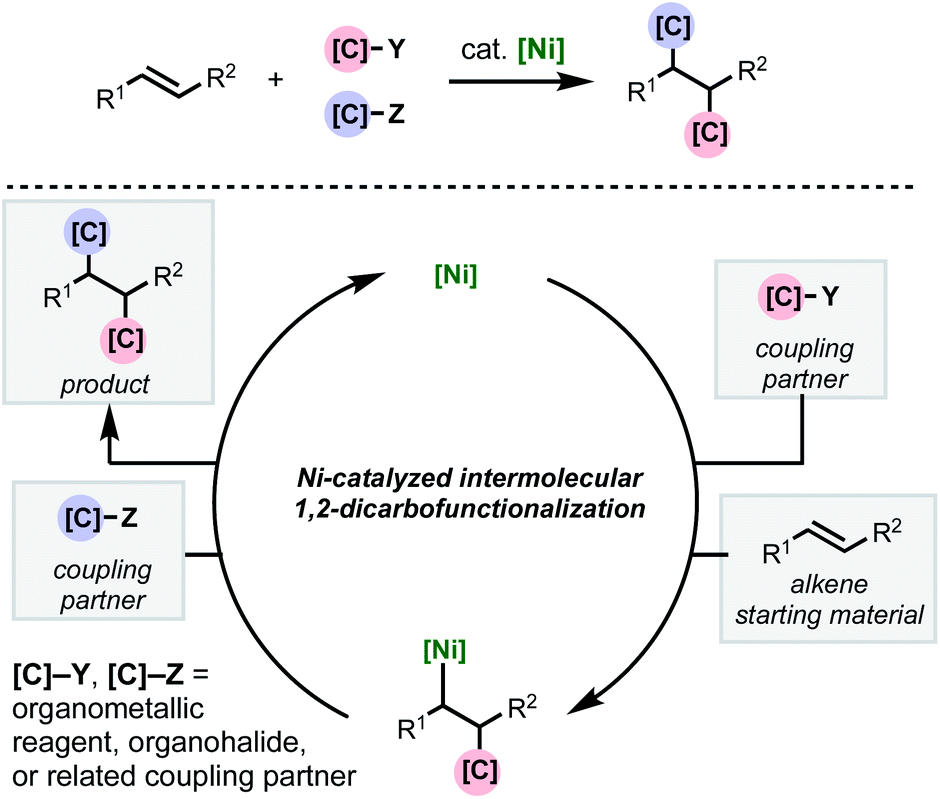
Recent developments in nickel-catalyzed intermolecular dicarbofunctionalization of alkenes - Chemical Science (RSC Publishing) DOI:10.1039/C9SC06006E

Ni(II)-Dimeric Complex-Derived Nitrogen-Doped Graphitized Carbon-Encapsulated Nickel Nanoparticles: Efficient Trifunctional Electrocatalyst for Oxygen Reduction Reaction, Oxygen Evolution Reaction, and Hydrogen Evolution Reaction | ACS Sustainable ...

Catalysts | Free Full-Text | Effect of the Gallium and Vanadium on the Dibenzothiophene Hydrodesulfurization and Naphthalene Hydrogenation Activities Using Sulfided NiMo-V2O5/Al2O3-Ga2O3

Mechanisms of Nickel-Catalyzed Coupling Reactions and Applications in Alkene Functionalization | Accounts of Chemical Research

An Unsaturated Nickel(0) NHC Catalyst: Facile Preparation and Structure of Ni(0)(NHC)2, Featuring a Reduction Process from Ni(II)(NHC)(acac)2 | Organometallics

An Unsaturated Nickel(0) NHC Catalyst: Facile Preparation and Structure of Ni(0)(NHC)2, Featuring a Reduction Process from Ni(II)(NHC)(acac)2 | Organometallics
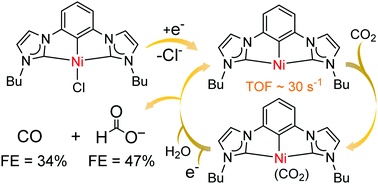
Electrocatalytic reduction of CO2 with CCC-NHC pincer nickel complexes - Chemical Communications (RSC Publishing)


