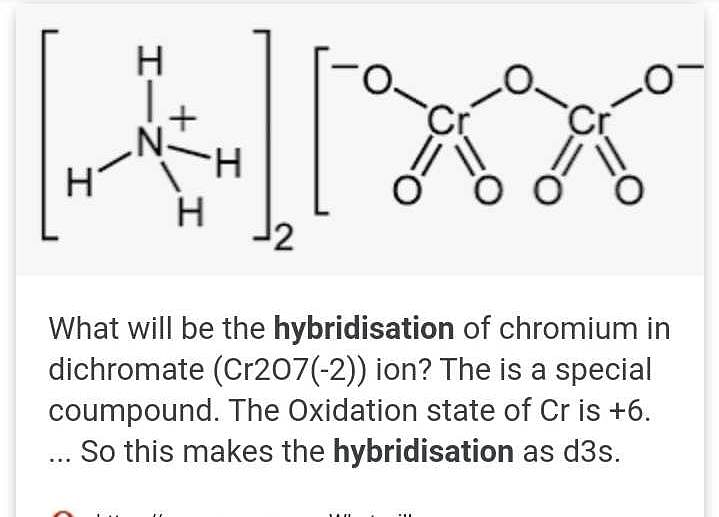
SOLVED: Balance the following reactions in an acidic solution (not all will need Ht ions) 1. Cr (s) + Ni2+ 7 Cr % (aq) + Ni (s) 2. Clz (aq) + Br

Scheme 1. Schematic illustration of the formation of Ag-Ni core-shell... | Download Scientific Diagram

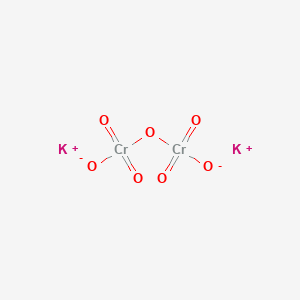
Chromium transition metal Chemistry chromium(III) Cr3+ complex ions chromate(VI) CrO42- dichromate(VI)Cr2O72- redox chemical reactions principal +3 +6 oxidation states ligand substitution GCE AS A2 IB A level inorganic chemistry revision notes
Color of Transition Metal Complexes The variety of color among transition metal complexes has long fascinated the chemists. For
Color of Transition Metal Complexes The variety of color among transition metal complexes has long fascinated the chemists. For

IB chemistry online - Transition elements-Colors: From left to right: Ti+2, V+3, VO2+, Cr+3, Cr2O7-2, Mn+2, MnO4-1, Fe+3, Co+2, Ni+2, Cu+2 | Facebook
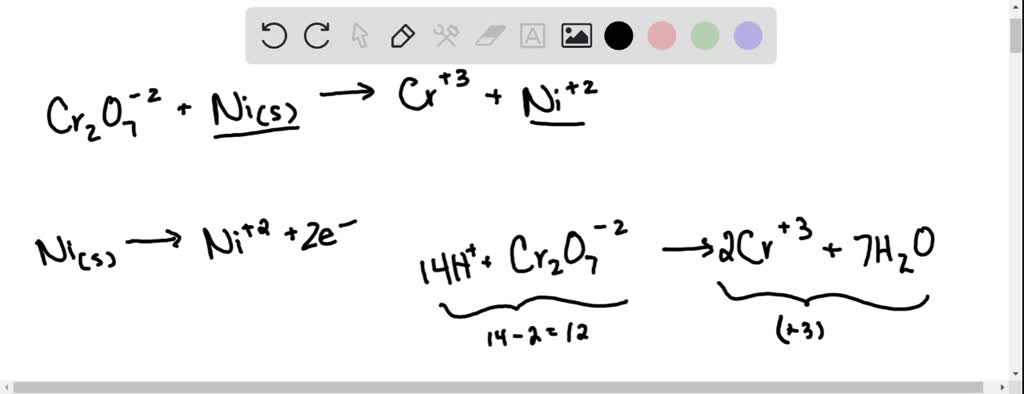
SOLVED: Balance the following redox equation in an acidic solution : Cr2O7 -2 (aq) + Ni (s) —– > Ni2+ (aq) + Cr3+ (aq) (acidic solution)
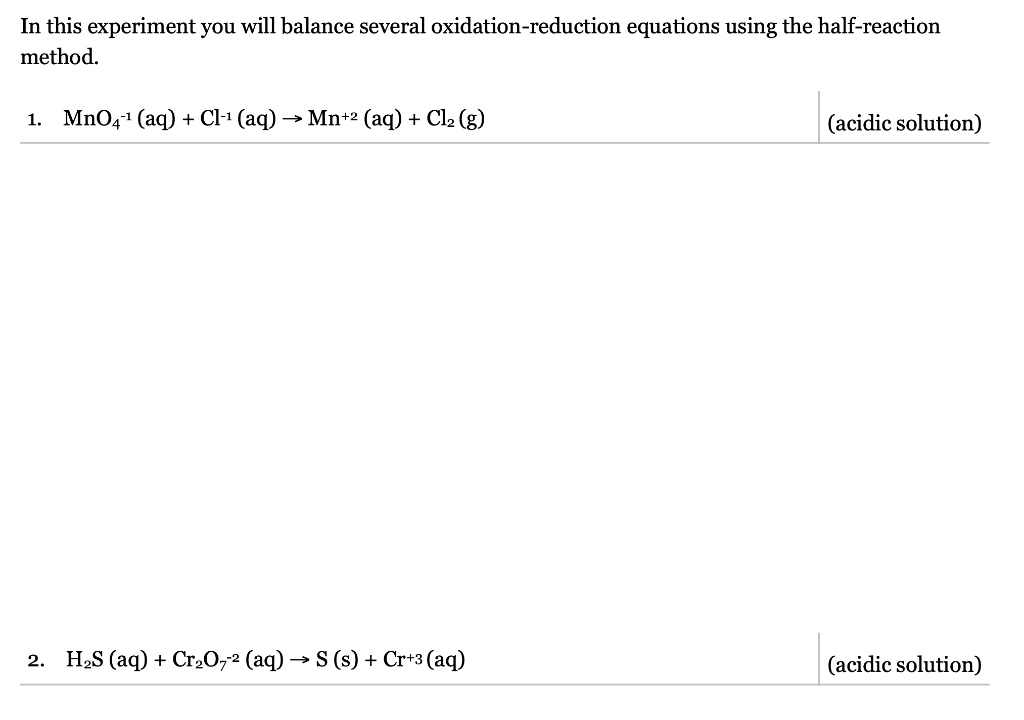
SOLVED: In this experiment you will balance several oxidation-reduction equations using the half-reaction method. 1.MnO4-1 (aq) + Cl-1 (aq) -> Mn+2 (aq) + Cl2 (g) (acidic solution) 2.H2S (aq)+ Cr2O7-2 (aq) ->

Calculate the equilibrium constant for the reaction: 3Sn(s) + 2Cr2O7^2 - + 28 H^+→ 3 Sn^+4 + 4Cr^3 + + 14H2O E^∘ for Sn/Sn^2 + = 0.136 V E^∘ for Sn^2 + /
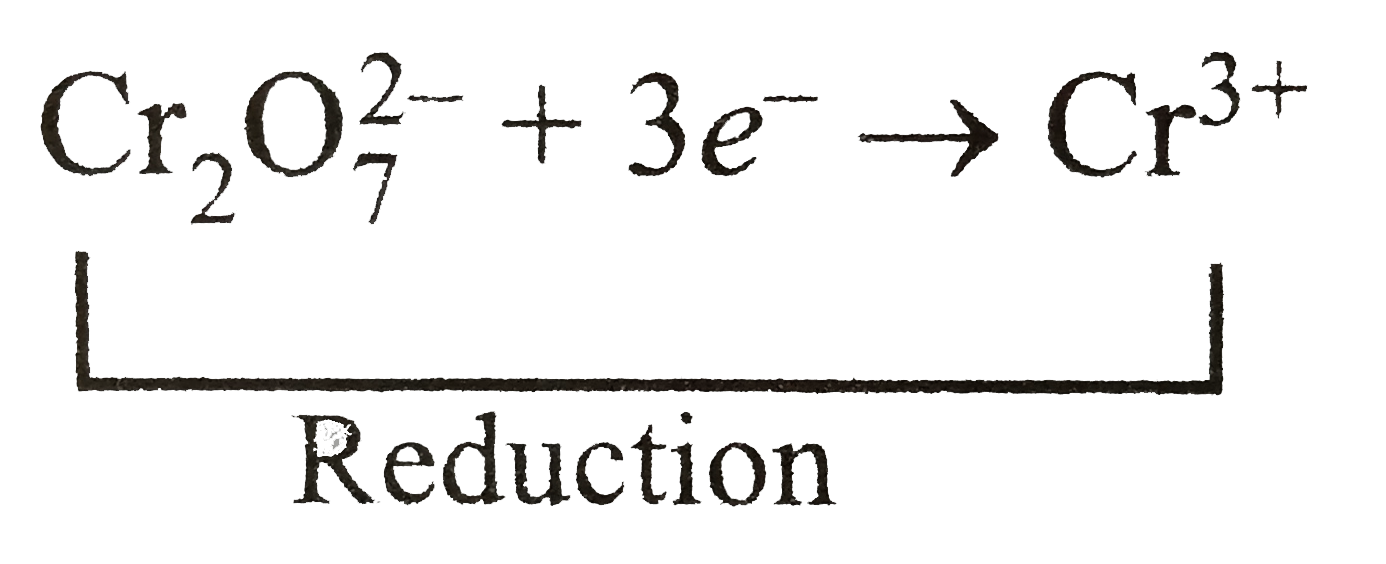









![PDF] Polar compounds constructed with the [Cr2O7]2- anion. | Semantic Scholar PDF] Polar compounds constructed with the [Cr2O7]2- anion. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/01bd3ffb43425676c7723ca53225f700e4d30c92/2-Table3-1.png)